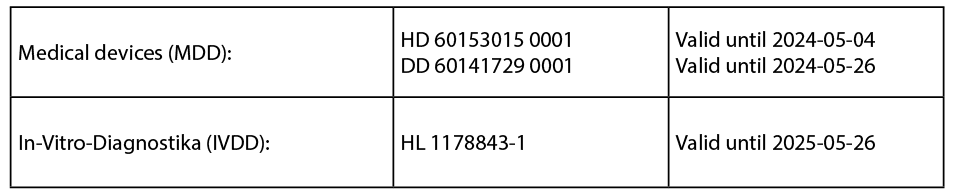
The best proof of the demanding MED TRUST quality management system are the years of certification according to EN ISO 13485:2016 and the CE certificates for our medical devices according to the EU Directives 93/42/EEC (MDD) and In Vitro Diagnostics 98/79/EC (IVDD) by TÜV Rheinland.
The EU Medical Device Regulation 2017/745 (MDR) and In Vitro Diagnostics Regulation 2017/746 (IVDR) set much higher standards for the Technical Documentation, clinical data, comprehensive reporting and notification requirements, among others, in order to be able to guarantee a high level of performance, safety and health protection.
Our dedicated MED TRUST Quality Management and Regulatory Team are continuously working on the Technical Documentation of our medical devices, thus proving that Wellion products fully comply with the increased requirements of CE conformity according to MDR and IVDR.
Registered Wellion products will be sold and remain on the market in compliance with the regulations until the end of the validity of the CE certificates (MDD/IVDD), even in the transition phase (MDR and IVDR "Grace Period").
Introduction of Unique Device Identification (UDI) in the form of a data matrix (unique product key)
Appointment of the Responsible Person (PRRC)
Complex market surveillance (including PMS, PSUR, vigilance, EUDAMED notification)
Adaptation of the quality management system:
Including risk management, post-market surveillance, market surveillance, vigilance, complaint management/ traceability (UDI), change managementScope for certification process with the Notified Body secured
MED TRUST as manufacturer
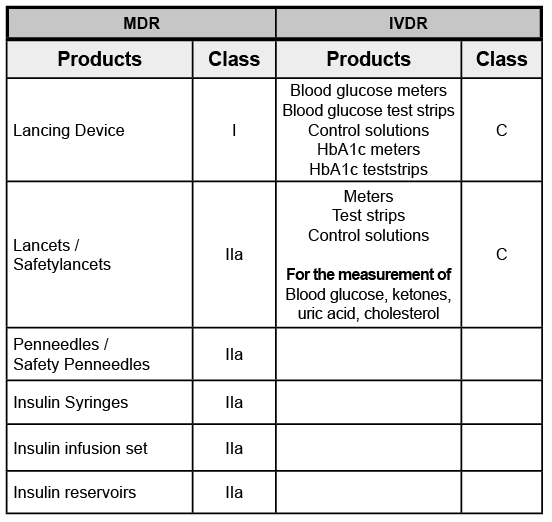
Portfolio of Wellion products:
MED TRUST as importer
Portfolio of private label Wellion products:
Blood pressure monitor
Nebulizer – Mesh Nebulizer
Thermometer
Pulse-Oximeter
Disposable syringe with low dead space
Cannulas / Safety Cannulas
SARS-CoV-2 antigen test
SARS-CoV-2 antibody test
Sensor (CGMS)Insulinpump system
https://www.medtrust.at/wellion/wellion-products/
FAQ's on the subject of MDR / IVDR at MED TRUST
What is the aim of the new EU regulations MDR and IVDR?
The background of the MDR or IVDR is the requirement for higher safety of products as well as their post-market follow-up. It should lead to better protection of public health and patient safety including higher transparency and traceability.
Which MED TRUST products are affected?
All medical devices and in vitro diagnostics.
What is the conformity assessment?
Medical devices are subject to CE marking and must have undergone a so-called conformity assessment procedure, which ultimately must be carried out not only by the manufacturer but also by the "so-called" Notified Body. The positive conclusion of such a procedure for the products is confirmed by the issuance of an EC certificate by the Notified Body.
What is a declaration of conformity?
The declaration of conformity is a document by which a manufacturer of a product declares on his own responsibility and in a legally binding manner (manufacturer's declaration of conformity) that its product complies with the requirements of the relevant EU directives.
Declarations of Conformity of the registered products are made available by MED TRUST for authorities and current inspections, as required by the MDR or IVDR, Article 14 "Obligations of distributors".
How can a customer (dealers, pharmacies, hospitals, partners, etc.) of MED TRUST products check the conformity of the products?
MED TRUST products clearly show the CE marking on the packaging.
www.certipedia.com/companies/573410/system_certificates
What do transition periods mean for MDR and IVDR?
If CE certificates have been issued in accordance with Directives 90/385 / EEC and 93/42 / EEC, they remain valid until the end of the period specified in the certificate. Provided that there are no significant changes, e.g. organisational changes, changes not related to the design and intended purpose. Parallel certifications are possible until 26 May 2020. The last MDD / IVDD certificates expire on 26 May 2024 and 26 May 2025 respectively and the products can be sold off for an additional year each.
What are the costs of MDR/IVDR at MED TRUST?
MED TRUST is continuously engaged in creating resources to ensure the timely implementation of the EU regulations. Investments in the millions are increasingly being made, with the main cost drivers being the creation of higher level Technical Documentation, increased post-market surveillance requirements, communication and auditing of outsourced processes, UDI labelling and warehouse logistics, among others.
Does MED TRUST have a quality management system that is constantly monitored?
Yes, MED TRUST is audited annually by TÜV Rheinland as its Notified Body. Furthermore, internal audits according to EN ISO 13485 are carried out in several areas, which ensures the further development of the quality management system according to the new EU requirements.
Does MED TRUST meet the requirements of the MDR/IVDR?
The Technical Documentation of the products has already been prepared and is continuously updated in accordance with the latest guidelines (MDCGs) and requirements of the Notified Body. The gradual transition of the products will take place until the maximum term of the CE certificates in 2024 resp. 2025.